Ozempic rocketed from a diabetes drug to a household weight-loss headline in just two years. Yet gastric bypass still delivers the most dramatic, long-term results doctors can offer. If you qualify for bariatric surgery—or want the strongest option—you’re weighing the convenience of a weekly shot against the permanence of an operation. In this guide, we stack weight-loss drugs vs surgery to compare results, risks, and costs so you can make an evidence-based choice. Ready? Let’s get started.
Ozempic: how a weekly shot reshapes appetite and metabolism
A balanced comparison of weekly GLP-1 injections and gastric bypass surgery as long-term weight-loss tools.
Ozempic is the brand name for semaglutide, a synthetic twin of the gut hormone GLP-1 that tells your brain you’re full. A once-weekly under-the-skin injection keeps semaglutide circulating for days, reducing appetite and slowing stomach emptying so meals feel satisfying longer.
Weight loss follows the chemistry. In the 2021 STEP 1 and STEP 3 trials, adults without diabetes shed 14.9 percent to 17.4 percent of their starting weight after sixty-eight weeks on the full 2.4 mg dose (sold as Wegovy). Real-world clinic data still show double-digit results—about 14 percent after one year—when patients stay on that target dose.
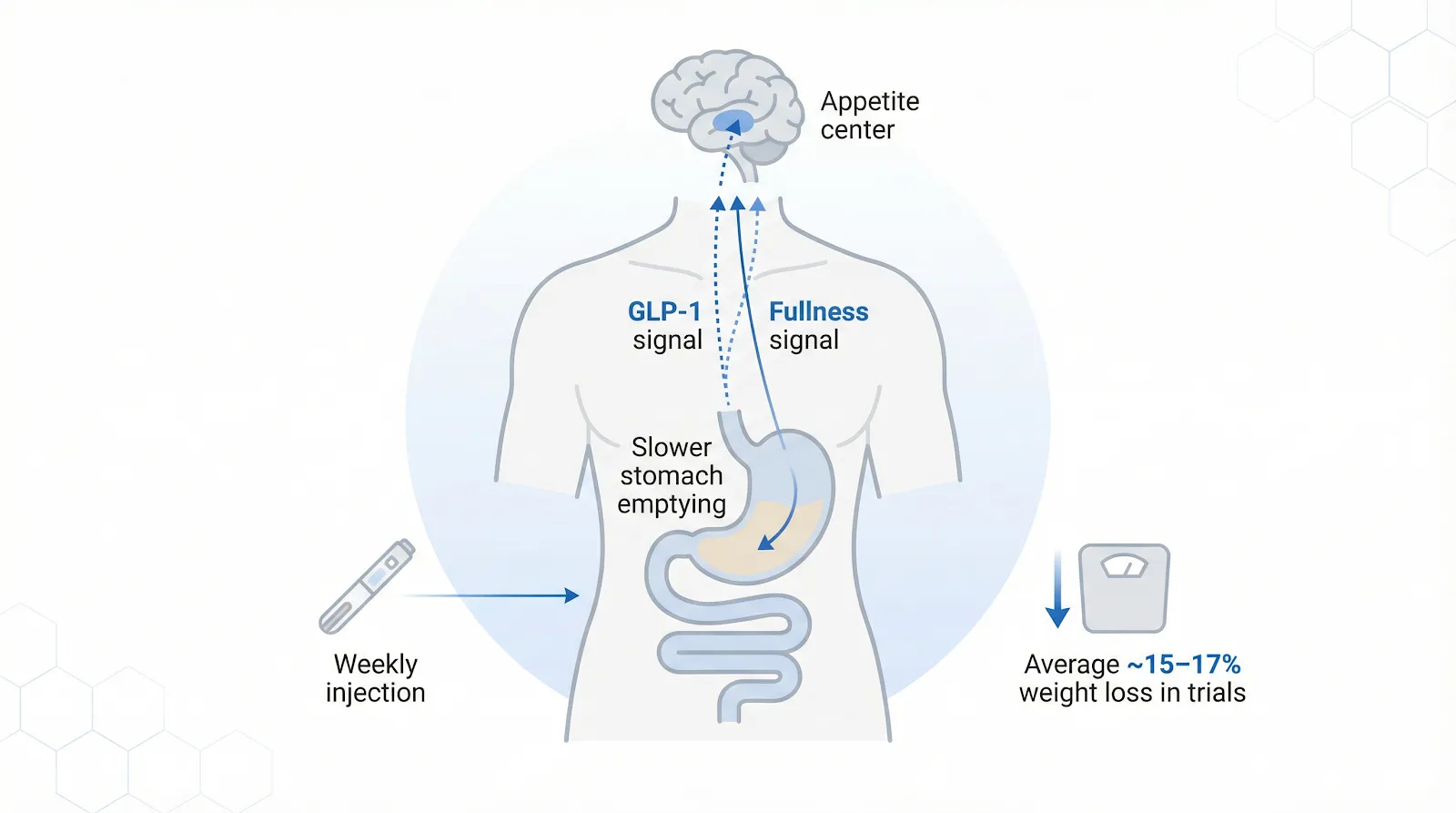
Semaglutide mimics the gut hormone GLP-1, signaling fullness in the brain and slowing stomach emptying to support weight loss.
The challenge is sticking with it. Digestive side effects remain common: pooled trial data found nausea in 43.9 percent, diarrhea in 29.7 percent, and vomiting in 24.5 percent of users, most often during dose escalation. Those symptoms prompt some people to pause increases or quit treatment, trimming the benefit.
Cost is another hurdle. As of December 2025, the list price runs about 1,350 dollars for a twenty-eight-day supply, or roughly 16,000 dollars per year without insurance. Coupons can drop self-pay prices to about 500 dollars at some United States pharmacies, yet coverage is uneven, and Medicare still bars obesity-only prescriptions.
When side effects are tolerable, and coverage cooperates, Ozempic offers a nonsurgical route to meaningful weight loss—provided you budget for a weekly pen and plan to stay on it for the long haul.
Gastric bypass is more than “shrinking the stomach.” Surgeons create a pouch about the size of an egg and connect it directly to the mid-jejunum. Food skips the remaining stomach and the first two feet of intestine, so you feel full sooner and absorb fewer calories.
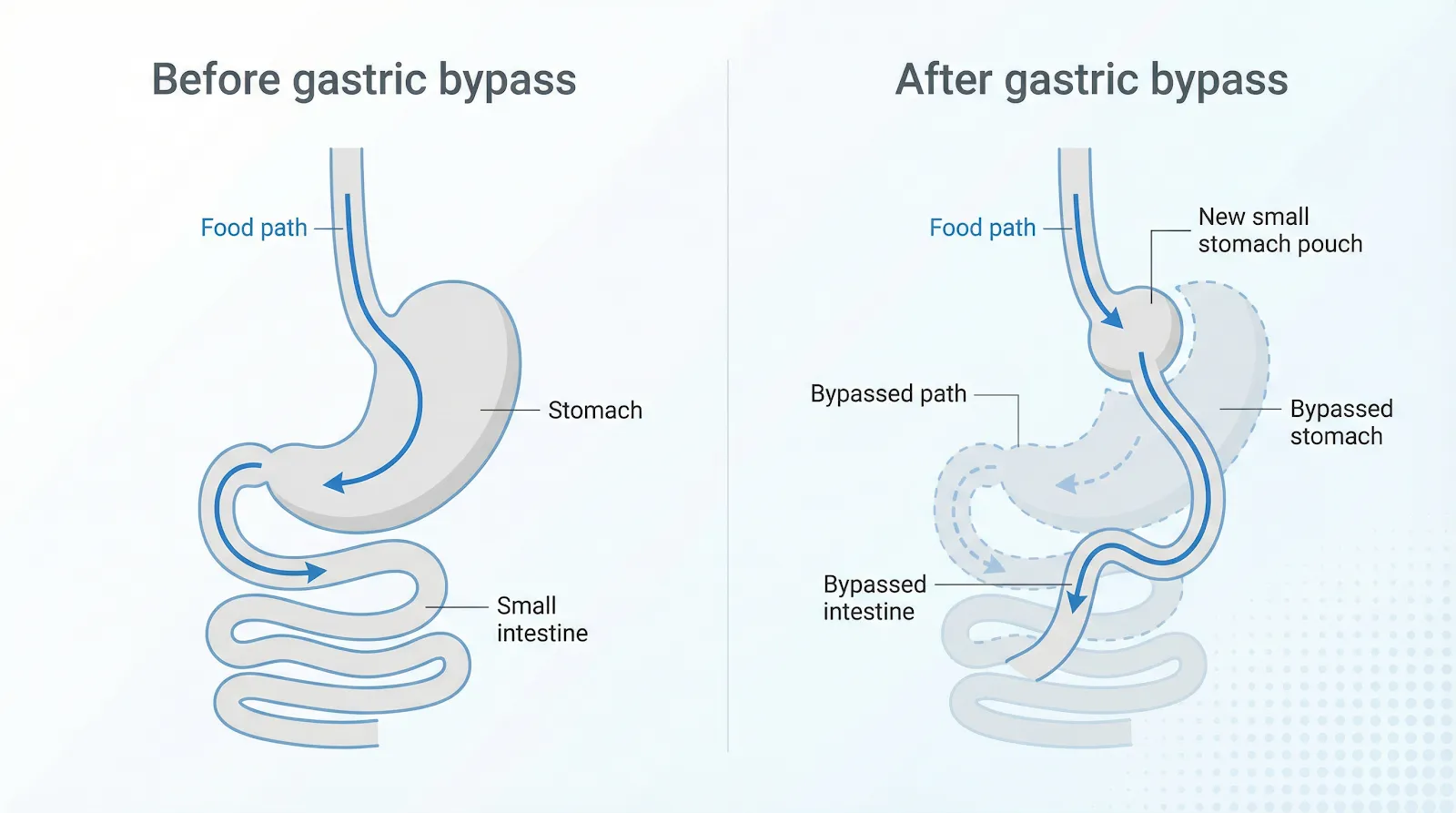
Gastric bypass creates a small stomach pouch and reroutes the intestine so food skips most of the stomach and upper bowel.
The operation is typically laparoscopic; five key-hole incisions while you sleep under general anesthesia. Most patients walk the hall the same day, leave the hospital in one to two days, and return to desk work within two weeks. The anatomy change is permanent.
Results are dramatic and durable. Large United States cohorts show patients lose about 30 percent of their starting weight at twelve months and keep roughly 25 percent off ten years later (analysis by the American Society for Metabolic and Bariatric Surgery). In a Veterans Affairs study published in JAMA Surgery, 72 percent of bypass patients still maintained at least 20 percent weight loss at ten years. Metabolic benefits often appear within days: blood sugar improves before major weight loss, and hypertension, sleep apnea, and joint pain frequently ease.
Commitment is essential. You’ll progress from clear liquids to purées, then to small, protein-focused meals over several months. Because the bypassed intestine absorbs nutrients less efficiently, lifelong multivitamins, iron, calcium, and B-12 are non-negotiable.
According to the Bariatric & Metabolic Center of Colorado’s patient guidelines, vitamin and protein levels are checked at least once a year for the first five years after malabsorptive procedures and supplements are adjusted based on those lab results.
That kind of structured follow-up keeps long-term risks like anemia, bone loss, and muscle loss low for most patients.
Risks remain, but are well quantified. Modern series report a 30-day mortality of 0.1 percent and major complications under 4 percent, numbers similar to gallbladder surgery. Early leaks, infections, or blood clots are uncommon and treatable. Later, dumping syndrome—a brief surge of nausea, palpitations, and fatigue after sugary food—serves as a natural brake on sweets.
In exchange, gastric bypass delivers a powerful metabolic reset and a lasting speed limit on overeating, benefits that persist long after the surgical scars fade.
Head-to-head results: which route drops more weight?
Numbers cut through hype, so let’s line the two approaches side by side.
Clinic-level reviews echo this national picture. In its side-by-side analysis of weight loss drugs vs surgery, the Bariatric & Metabolic Center of Colorado reports that second-generation GLP-1 shots trim roughly 12 - 18 percent of body weight, while bariatric operations regularly remove 65 - 100 percent of excess pounds—a gulf we’ll explore below.
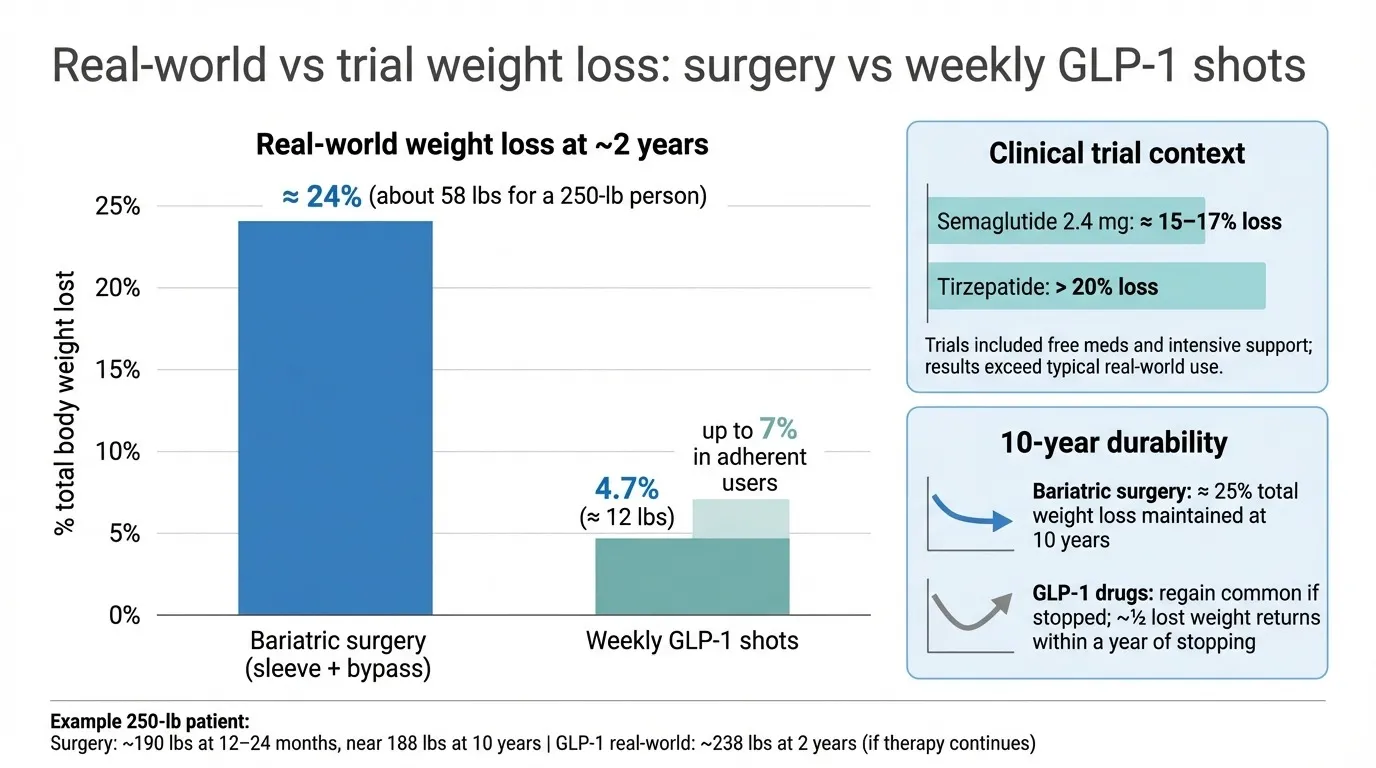
Bariatric surgery typically produces far greater and more durable weight loss than weekly GLP-1 shots, especially over ten years.
Real-world evidence. A study of 9,902 patients presented at the American Society for Metabolic and Bariatric Surgery in June 2025 found that sleeve gastrectomy and gastric bypass delivered 24 percent total weight loss—about 58 pounds—after two years. Patients prescribed weekly GLP-1 shots lost 4.7 percent, roughly 12 pounds, even when they filled prescriptions for at least six months. Those who stayed on therapy for a full year reached 7 percent, yet still lagged far behind surgery.
Clinical-trial context. In tightly controlled studies, semaglutide 2.4 milligrams trimmed 14.9 to 17.4 percent of body weight, while tirzepatide hit more than 20 percent. Those trials provided free medication, one-on-one coaching, and no insurance hurdles—conditions that are rare in everyday practice.
Durability. Bariatric surgery’s edge widens over time: registries show patients maintaining about 25 percent total weight loss at ten years and keeping obesity-related diseases in remission. By contrast, weight regain follows drug discontinuation; half the lost pounds typically return within a year of stopping GLP-1 therapy, according to multiple follow-up analyses.
Putting the math on a real body. For a 250-pound adult:
Gastric bypass: about 190 pounds after twelve to 24 months, still near 188 pounds a decade later
Ozempic (real world): about 238 pounds at two years, and only if injections and insurance coverage continue
That gulf explains why surgeons still call bypass the gold standard, while obesity-medicine physicians hail GLP-1s as the first drug class to put a dent in that gold.
Risks and side effects: weighing safety on both paths
Every medical fix carries a cost beyond dollars. With Ozempic, the price shows up as weekly needles and digestive discomfort. In pooled trials, nausea affected 43.9 percent, diarrhea 29.7 percent, and vomiting 24.5 percent of users, mostly during dose escalation. Symptoms fade for many within two months, yet roughly one in five people stop the drug within a year because of side effects or expense.
Less common but serious issues can appear. Rapid weight loss may trigger gallstones; pancreatitis and, rarely, bowel obstruction have been reported. The FDA label also flags thyroid C-cell tumors seen in rodents, so clinicians avoid GLP-1s if your family carries that risk. Recent studies note that 30 to 40 percent of pounds lost may come from lean muscle, prompting advice to add resistance training.
Gastric bypass carries greater upfront danger but fewer daily annoyances once wounds heal. Modern case series report a 30-day mortality of 0.1 percent and major complications under 4 percent, numbers similar to gallbladder surgery. Early leaks, infections, or clots usually surface in the first week while the care team watches closely.
Long term, nutrition takes center stage. Skipping daily supplements can lead to iron-deficiency anemia, vitamin B-12 shortage, or bone loss. Internal hernias or ulcers may appear years later, but are uncommon and treatable. Dumping syndrome—brief nausea, palpitations, and fatigue after sugary food—serves more as a built-in speed bump than a medical crisis.
So which path feels safer? Needles seem gentle today, but their side effects persist as long as the prescription, and stopping often means weight regain. Surgery requires a bold, one-time leap, then shifts to small meals, vitamin gummies, and yearly labs. Safety is not a scoreboard; it unfolds over time. Decide whether you prefer to front-load risk in an operating room or spread it across years of injections, and the answer becomes clearer.
Who qualifies? choosing the right tool for your BMI and health
Bariatric surgery: when does the OR door open?
The 2022 ASMBS and IFSO guidelines recommend metabolic or bariatric surgery for:
Body mass index of at least 35 kilograms per square meter at any time
Body mass index 30 to 34.9 with type 2 diabetes or another serious metabolic disease that is not controlled by medication
Adolescents and many Asian adults are at slightly lower thresholds because complications develop at lower weights
You will also need to:
Complete a medical work-up that confirms you can tolerate anesthesia
Show commitment to lifelong follow-up with diet, supplements, and lab work
Meet any insurance requirements, such as documented attempts at supervised weight-loss programs
Ozempic, Wegovy, and other GLP-1 drugs: who earns a prescription?
The United States Food and Drug Administration approved semaglutide 2.4 milligrams (Wegovy) for adults with:
Body mass index of at least 30, or
Body mass index at least 27 plus a weight-related condition such as hypertension, dyslipidemia, or sleep apnea
Clinicians avoid GLP-1s in people with a personal or family history of medullary thyroid cancer, multiple endocrine neoplasia type 2, or recurrent pancreatitis. Coverage varies: Medicare still excludes obesity-only prescriptions, while some private plans now cover GLP-1s for cardiovascular risk reduction.
How guidelines are shifting
A 2025 consensus from the European Association for the Study of Obesity argues that GLP-1 drugs should be first-line therapy for many patients, reserving surgery for those who do not respond to medication or need rapid relief. United States societies counter that only about 1 percent of eligible Americans ever reach the operating room and urge earlier surgical referral when BMI and comorbidities justify it.
Blending the two
Many centers now combine tools:
Before surgery. Short courses of semaglutide can shrink the liver by about 15 to 20 percent in a few weeks, lowering operative risk
After surgery. GLP-1s or tirzepatide can help curb weight regain years later
Eligibility, then, is not a single choice but a toolbox. A coordinated team of a surgeon, obesity-medicine physician, a dietitian, and psychologist can match the right tool—or sequence of tools—to your BMI, health profile, and insurance realities.
Cost and insurance: the long game for your wallet
Money whispers in every exam room, sometimes louder than medical advice.
Medication math. Wegovy’s list price runs about 1,350 dollars for a 28-day supply, roughly 16,200 dollars per year without insurance or discounts. Manufacturer coupons can reduce early fills to zero to 349 dollars a month, and some telehealth programs advertise introductory pricing as low as 199 dollars. Coverage is uneven: many commercial plans require prior authorization, and in April 2025, the Centers for Medicare and Medicaid Services confirmed that Medicare Part D will not cover GLP-1s used solely for obesity. A five-year supply paid in cash can exceed 80,000 dollars even after coupons.
Surgery sticker shock. Gastric bypass averages 17,000 to 26,000 dollars in the United States. Insurers often cover it once you meet BMI criteria, leaving deductibles and copays of a few thousand dollars for many patients. Self-pay packages abroad can dip below 10,000 dollars, but add travel and follow-up costs.
Upkeep costs diverge. Drugs require continuous payment; stop the pen, and weight often returns. Surgery swaps needles for pills: multivitamins, calcium, and annual labs usually cost 300 to 500 dollars a year. Plastic-surgery or complication expenses are possible but uncommon, and studies show insurers recoup the upfront surgical expense within two to four years thanks to lower spending on diabetes, sleep-apnea, and hypertension care.
Five-year snapshot.
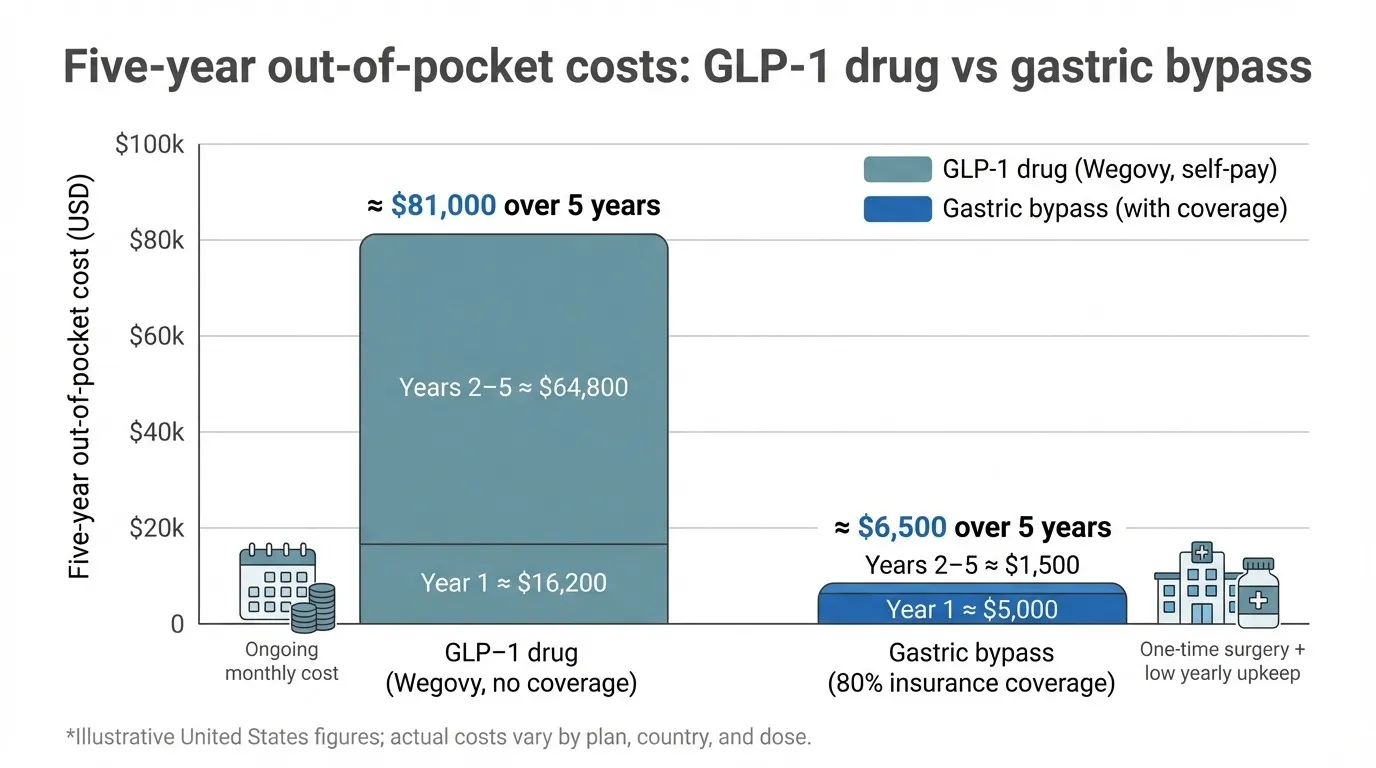
Over five years, self-paid Wegovy injections can cost more than ten times an insured gastric bypass plus supplements.
Path | Year-1 out-of-pocket* | Years 2 to 5 combined | Five-year total |
Wegovy (no coverage) | about 16,200 dollars | about 64,800 dollars | about 81,000 dollars |
Gastric bypass (with 80 percent insurance coverage) | about 5,000 dollars in deductible and copay | about 1,500 dollars for vitamins and labs | about 6,500 dollars |
*Illustrative United States figures; actual costs vary by plan and dose.
Bottom line: Surgery front-loads the bill but often wins the marathon when insurance helps. Drugs spread expenses over time and stay affordable only when a plan or coupon shoulders most of the monthly tab. Decide which cash-flow pattern and which health payoff fits your long game.
Lifestyle impact: living with each choice day to day
Life after gastric bypass. Surgery changes your relationship with food overnight. During weeks 1 to 2, you focus on clear liquids and protein shakes; most programs aim for 60 to 80 grams of protein each day to protect muscle. By month three, you are eating toddler-size meals: lean protein first, vegetables next, carbohydrates last. A single extra bite often causes discomfort, reinforcing portion control. Alcohol feels stronger because it is absorbed faster, and sugary drinks may trigger dumping syndrome, so label reading becomes second nature. Vitamins sit on the nightstand, and a daily walk usually grows into longer cardio sessions as energy returns.
Life on Ozempic or Wegovy. Your anatomy stays intact, so your favorite foods remain on the menu, but smaller portions feel satisfying. Many users time the weekly shot before bed or on Friday to sleep through mild nausea. Because 30 to 40 percent of drug-related weight loss can come from lean muscle without strength work, lifting weights twice a week is essential. A sugar-free ginger ale in the fridge often helps settle queasy moments.

Gastric bypass and GLP-1 shots reshape daily life in different ways, from tiny meals and vitamins to weekly injections and strength training.
Motivation curves differ. Post-operative pounds drop quickly, which can supercharge adherence when temptation strikes. Drug-based loss moves steadily; progress photos and smart-scale trends help you stay patient. Either way, long-term success rests on four habits: whole foods, regular movement, stress control, and scheduled follow-ups. The tool opens the door, and your daily choices decide how far you walk through it.
Emerging trends and the road ahead
Pharmaceutical innovation is accelerating. Just behind semaglutide comes tirzepatide, a dual GLP-1 and GIP agonist that cut 22 to 24 percent of body weight at 72 weeks in the SURMOUNT-1 and SURMOUNT-2 trials (New England Journal of Medicine, 2022–2023). Third-generation candidates are already in phase 2. GLP-1, GIP, and glucagon “tri-agonists” such as retatrutide produced about 24 percent loss in 48 weeks, and early studies are pairing GLP-1s with myostatin inhibitors to protect muscle.
Durability remains the key question. An ASMBS 2024 review found gastric-bypass patients maintaining about 25 percent total weight loss a decade later, while most drug studies end after two years. Until pills prove similar staying power, surgery keeps its edge for permanence.
Care models are shifting from either-or to both-and. Many centers start high-BMI patients on semaglutide for a month to shrink liver volume by 15 to 20 percent, lowering operative risk, then reserve GLP-1s or tirzepatide to curb weight regain years after bypass.
Policy could widen access further. A Nature Medicine consensus in October 2025 recommended GLP-1 drugs as first-line therapy for many patients. In the United States, the Treat and Reduce Obesity Act of 2025 would pilot Medicare coverage for anti-obesity medication. Early cost-effectiveness studies, however, still favor surgery when insurance already covers it.
What does this mean for you? The treatment toolbox is expanding. Future plans may combine short-term liver-shrinking medication, minimally invasive sleeves, and maintenance injections—chosen to match your biology, budget, and timeline. One fact stays constant: results last only when the tool you choose fits into sustainable daily habits.
लेखक





